Lives on the Line: Lawsuits Over Defective Medical Instruments
High-stakes courtroom battles are raising alarms about patients harmed by faulty surgical tools. Each case underscores the critical need for reliable quality control in instrument manufacturing.
1. Blade Tip Lodges Inside Patient During Hysterectomy (UK, Oct 2023)
A 44-year-old woman, Jane Harvey, is suing an NHS trust after the tip of a surgical blade broke off during her abdominal hysterectomy and went unnoticed—even on X-ray. She endured a second operation, extended hospitalization, and severe anxiety (nightmares, panic, loss of appetite). The surgeon attributed the blade failure to a manufacturing defect (theguardian.com).
2. Retained Plastic Trocar Tips in Salpingectomy (Florida, 2022)
In another alarming case, a plastic trocar used during laparoscopic surgery fractured, leaving pieces inside the body. This led to bowel perforation, life-threatening organ failure, multiple surgeries, and ongoing sepsis treatment (medicalmalpracticelawyers.com).
3. Defective Surgical Stapler – Florence Kuhlmann (California, 2012)
During a hemorrhoidopexy in January 2012, a Proximate PPH‑03 stapler malfunctioned—it misfired and sealed the patient’s bowel incorrectly. The result: emergency hospitalization, severe infections, multiple subsequent surgeries, and a lasting colostomy. In December 2015, a jury awarded $9.8 million in compensatory damages and $70 million punitive—a total of nearly $80 million (aorn.org). The verdict was upheld on appeal, though punitive damages were partially reduced to ~$19.6 million .
4. Unapproved Equipment, Fatal Sepsis Post-Hysterectomy (UK, 2023)
Jessica Bonner, 51, tragically died from sepsis after a hysterectomy at Good Hope Hospital when unapproved surgical equipment was used and a perforated bowel went undetected. A coroner ruled her death preventable, noting “missed opportunities” and equipment failures (thesun.co.uk).
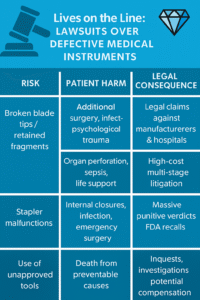
Common Patterns & Lessons
| Instrument & Defect | Patient Harm | Legal Consequences |
|---|---|---|
| Broken blade tips / retained fragments | Additional surgery, infection, psychological trauma | Legal claims against manufacturers and hospitals |
| Trocar tip failures | Organ perforation, sepsis, life support | High-cost multi-stage litigation |
| Stapler malfunctions | Internal closures, infection, emergency surgery | Massive punitive verdicts, FDA recalls |
| Use of unapproved tools | Death from preventable causes | Inquests, investigations, potential compensation |
Why This Matters — & How Rigor Instruments Can Lead the Way
These cases reveal a stark reality: patients are suffering because of manufacturing defects, insufficient inspection, inadequate design validation, and unauthorized tools in the OR. Beyond the human toll, institutions face devastating legal costs, reputational damage, and loss of trust.
Our mission at Rigor Instruments is more than a business—it’s a life-saving imperative. Every instrument you produce with uncompromising quality, strict testing, and full certification helps prevent another tragic headline.
How Rigor Instruments Educates Its Inspection Team Using Real Lawsuits & Case Studies
1. Case-Based Training Modules
Rigor has developed internal training that includes:
Real-life lawsuit breakdowns (e.g. broken blades, trocar tip retention, stapler failures)
Visuals of defects that caused harm in the past
Root cause analysis of each case, highlighting what inspectors must look for (e.g. cracks, weld failures, misalignment, contamination)
“If it’s happened before in the industry, it’s our duty to prevent it from happening again.”
2. Simulated Fault Detection Exercises
QA staff go through regular simulation tests:
Fake defected batches based on previous lawsuits
Required to identify defects within set timelines
Errors are debriefed and used for team improvement
3. Legal & Ethical Responsibility Workshops
The team is trained not just in mechanics but in ethics:
Understanding the legal implications of poor inspections
How patient lives are impacted
Stories of real patients who suffered due to bad tools
This builds empathy, not just efficiency.
4. Checklists Enhanced by Lawsuit Learnings
Every inspection checklist at Rigor is continuously improved based on:
FDA/CE recall data
Legal case outcomes
Surgeon feedback on real-world failures
For example:
“Retained part check” was added after trocar lawsuits.
Tip sharpness stress test reinforced after hysterectomy blade failure case.
5. Monthly “Failure Briefs” — Quality Bulletins
Every month, the QA team receives a bulletin:
Top 5 industry failures from global sources
Actions taken by Rigor to proactively mitigate such risks
Internal scorecards tracking Rigor’s QA success
6. ISO 13485 & CE Training + Internal Audits
Rigor ensures:
All staff are trained per ISO 13485:2016 guidelines
Mock audits simulate CE compliance inspections
Any team member can be tested on their knowledge of why standards matter
Final Word:
“At Rigor, our QA inspectors aren’t just checking boxes—they’re protecting patients. Every lawsuit out there is a lesson we engrain into our system to ensure that no patient ever suffers from a mistake we could have caught.”